Electron Microscopy
Detailed investigation of synapse morphology is necessary to understand how changes in synapses relate to learning and the formation of memory. Electron microscopy (EM) can be used to study the synaptic basis for neural circuits, and is currently the only available technique which images at a high enough resolution to visualize synapse ultrastructure. It was this technique which first allowed for the characterization of fine synaptic structure in the 1950's (Gray, 1959; Palay and Palade, 1955). Electron microscopy involves the use of an electron beam which is shone onto a sample prepared for EM. This sample has to be fixed and incubated in electron dense solutions to increase contrast in typically transparent biological specimens. Based on the electron density of regions within the fixed sample, electrons may pass through the sample or deflect off the surface, or cause secondary electrons to be emitted by the specimen.
Focused Ion Beam Scanning Electron Microscopy (FIB-SEM) is an advanced type of electron microscopy which allows one to achieve higher resolution in the z-plane. This is accomplished by milling a thin section from the surface of a sample using an ion beam. The precision of the (gallium) ion beam allows for sections to be as thin as 3nm, much thinner than those obtained by conventional electron microscopy techniques (reviewed by: Kizilyaprak et al., 2014). This results in voxel resolution high enough to generate electron microscopy volumes, so that the three-dimensional characteristics of objects can be visualized and investigated.
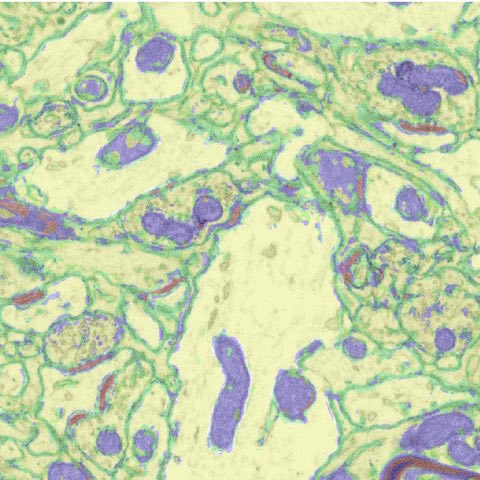
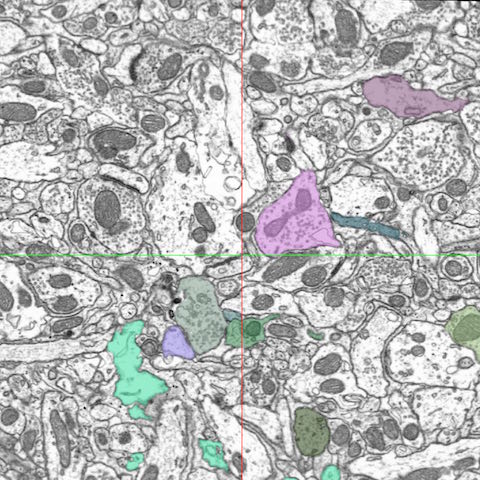
While FIB-SEM has been in use for the study of biological samples since at least 1995 (Ishitani et al.), this technique is still relatively unexplored in the field of neuroscience (ex. Blazquez-Llorca et al., 2013; Khanmohammadi et al., 2015; Maco et al., 2014) despite a recent abundance of techniques facilitating semi-automated analysis of synapses within FIB-SEM volumes (Kreshuk et al., 2011; Morales et al., 2011). In light of this, we are taking advantage of this advanced technology to investigate the ultrastructural changes that occur in the songbird brain as a result of song learning.
Recent studies have shown that dendritic spine size and stability in the
zebra finch HVC is associated with song exposure (Tschida and Mooney, 2012;
Roberts et al 2010). In other model systems, it has been shown that stronger
synapses are correlated with larger and more stable spines (Grutzendler et al.,
2002, Gilbert and Soderstrom, 2013, Kuwajima et al., 2013). Moreover, spine
shape has been correlated with synapse strength and stability
(Bosch et al., 2015). In regards to song learning in zebra finches, one
study showed that deafening led to a decrease in dendritic spine size
specific to HVCX neurons (Tschida and Mooney, 2012). We are now investigating
the ultrastructural changes that occur at synapses in the HVC upon exposure
to tutor song, such as changes in morphology of pre and post-synaptic targets,
post-synaptic densities (PSDs) and connectivity. Using FIB/SEM images, we
are using several 3D rendering programs for data analysis and reconstructions
(such as Ilastik, Neuromorph and TrackEM) to investigate changes in bouton
size, neuronal classification (symmetric or asymmetric synapses, Multi
Synaptic Boutons, en passant or terminaux boutons) and mitochondrial
morphology associated with tutor song exposure.
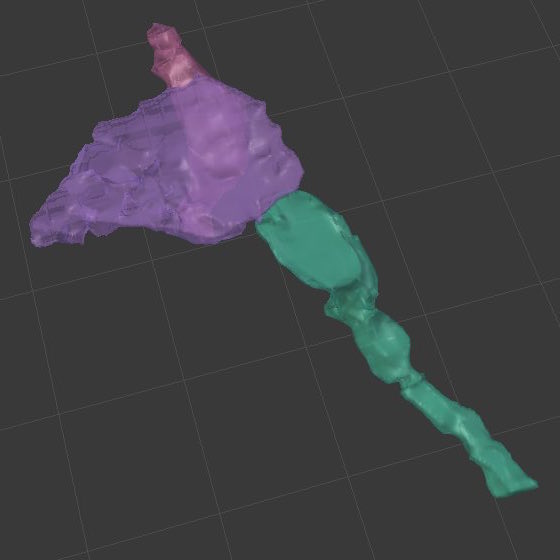
A) 3-D reconstruction of a terminaux bouton in Blender as a multiple synapse bouton. Terminaux boutons are presynaptic terminals found on axonal branches and boutons that synapse on dendritic shafts to establish symmetric synapses, which usually have inhibitory effects and are generally less prevalent (reviewed by: Holtmaat & Svoboda 2009).
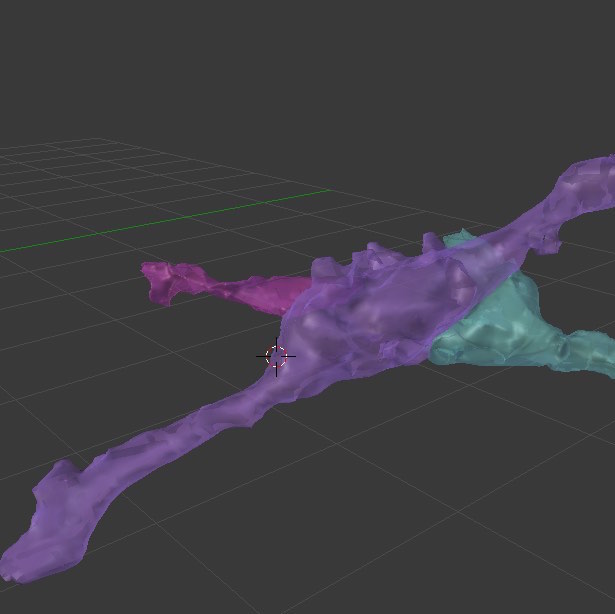
B) 3-D reconstruction of an en passant bouton in Blender as a multiple synapse bouton. En passant boutons are axonal varicosities that tend to form excitatory asymmetric synapses on dendritic spines (reviewed by: Holtmaat & Svoboda, 2009).
References:
- Blazquez-Llorca, L., Merchan-Perez, A., Rodriguez, J.R., Gascon, J., and DeFelipe, J. (2013). FIB/SEM technology and Alzheimer's disease: three-dimensional analysis of human cortical synapses. J Alzheimers Dis 34, 995-1013.
- Bosch C, Martínez A, Masachs N, Teixeira CM, Fernaud I, Ulloa F, Pérez E, Lois C, Comella JX, DeFelipe J, Merchan-Perez A, Soriano E (2015) FIB/SEM technology and high-throughput 3D reconstruction of dendritic spines and synapses in GFP-labeled adult-generated neurons. Frontiers in Neuroanatomy 9.
- Gilbert MT, Soderstrom K (2013) Novel song-stimulated dendritic spine formation and Arc/Arg3.1 expression in zebra finch auditory telencephalon are disrupted by cannabinoid agonism. Brain research 1541:9-21.
- Gray, E.G. (1959). Axo-somatic and axo-dendritic synapses of the cerebral cortex: an electron microscope study. J Anat 93, 420-433.
- Grutzendler J, Kasthuri N, Gan W-B (2002) Long-term dendritic spine stability in the adult cortex. Nature 420:812-816.
- Holtmaat, A., and Svoboda, K. (2009). Experience-dependent structural synaptic plasticity in the mammalian brain. Nature reviews Neuroscience 10, 647-658.
- Ishitani, T., Hirose, H., and Tsuboi, H. (1995). Focused-ion-beam digging of biological specimens. J Electron Microsc (Tokyo) 44, 110-114.
- Jorstad, A., Nigro, B., Cali, C., Wawrzyniak, M., Fua, P., and Knott, G. (2015). NeuroMorph: a toolset for the morphometric analysis and visualization of 3D models derived from electron microscopy image stacks. Neuroinformatics 13, 83-92.
- Khanmohammadi, M., Waagepetersen, R.P., and Sporring, J. (2015). Analysis of shape and spatial interaction of synaptic vesicles using data from focused ion beam scanning electron microscopy (FIB-SEM). Frontiers in neuroanatomy 9, 116.
- Kizilyaprak, C., Daraspe, J., and Humbel, B.M. (2014). Focused ion beam scanning electron microscopy in biology. Journal of microscopy 254, 109-114.
- Kreshuk, A., Straehle, C.N., Sommer, C., Koethe, U., Cantoni, M., Knott, G., and Hamprecht, F.A. (2011). Automated detection and segmentation of synaptic contacts in nearly isotropic serial electron microscopy images. PloS one 6, e24899.
- Kuwajima M, Spacek J, Harris KM (2013) Beyond counts and shapes: studying pathology of dendritic spines in the context of the surrounding neuropil through serial section electron microscopy. Neuroscience 251:75-89.
- Maco, B., Holtmaat, A., Jorstad, A., Fua, P., and Knott, G.W. (2014). Correlative in vivo 2-photon imaging and focused ion beam scanning electron microscopy: 3D analysis of neuronal ultrastructure. Methods Cell Biol 124, 339-361.
- Morales, J., Alonso-Nanclares, L., Rodriguez, J.R., Defelipe, J., Rodriguez, A., and Merchan-Perez, A. (2011). Espina: a tool for the automated segmentation and counting of synapses in large stacks of electron microscopy images. Frontiers in neuroanatomy 5, 18.
- Palay, S.L., and Palade, G.E. (1955). The fine structure of neurons. J Biophys Biochem Cytol 1, 69-88.
- Roberts, T.F., Tschida, K.A., Klein, M.E., and Mooney, R. (2010). Rapid spine stabilization and synaptic enhancement at the onset of behavioural learning. Nature 463, 948-952.
- Tschida KA, Mooney R (2012) Deafening drives cell-type-specific changes to dendritic spines in a sensorimotor nucleus important to learned vocalizations. Neuron 73:1028-1039.