pH
The pH of a solution
is a measure of its acidity. The pH is
defined as the
negative log of the hydrogen ion concentration. The
pH scale ranges from 0 to 14 where 0
is the most acidic, 14
is the most basic, and 7 is neutral.
Remember that since pH is a log
scale,
the difference between any two numbers
is a factor of
10, so precise measurement
of pH is very important. In the lab,
pH can be measured with a pH meter or
pH paper.
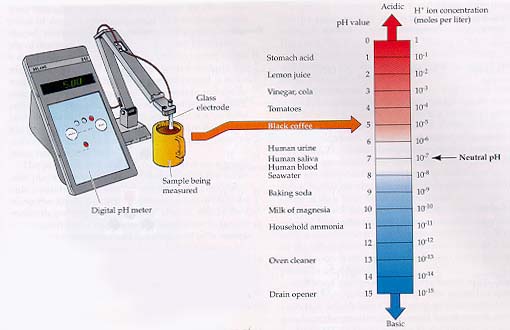 pH values of some
familiar substances (from Purves et al 1998)
pH values of some
familiar substances (from Purves et al 1998)